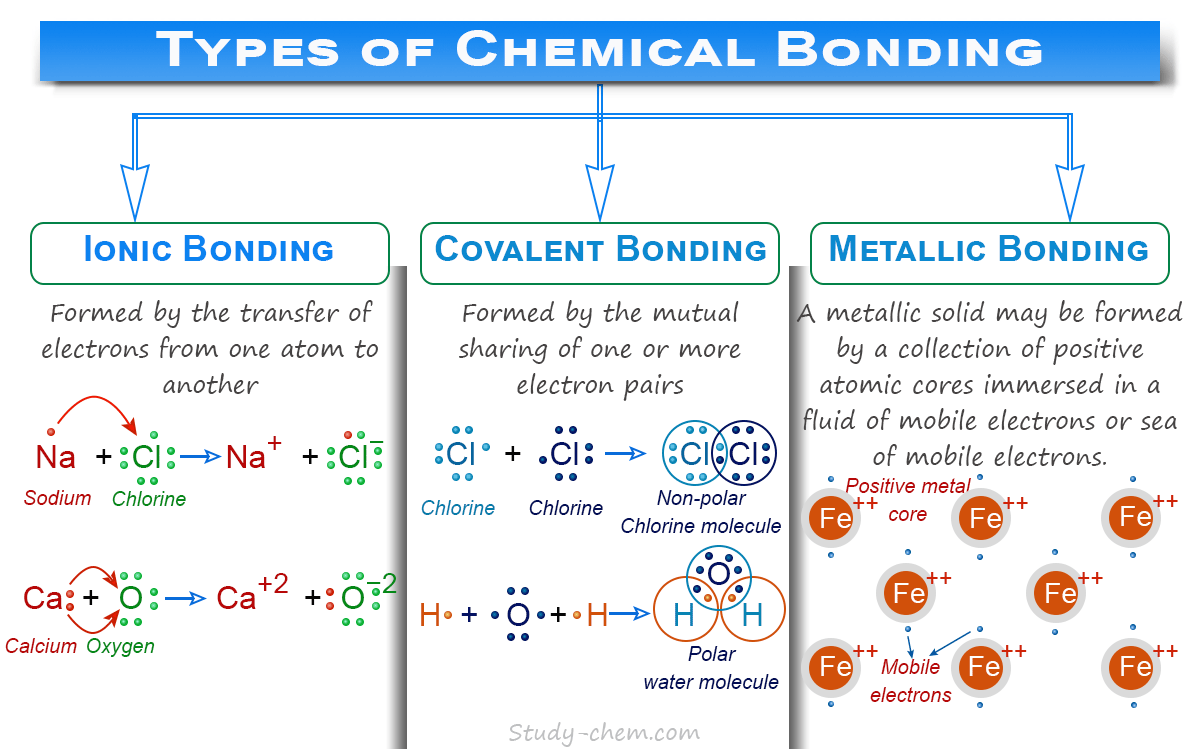
Definition of Chemical Bond
A chemical bond is the force of attraction between the atoms, ions, or molecular species that enables the formation of chemical compounds by lowering energy. Such a definition of the chemical bond may be found and discussed under the three common classes: ionic, covalent, and metallic bonding. An ionic bond or ionic bonding is formed by the electrostatic force of attraction between oppositely charged ions (cations and anions). However, a covalent bond is formed through the sharing of electrons.
Ionic Bond
An ionic bond or electrovalent bond is formed when one or more electrons from the valence shell of an atom are completely transferred to the valence shell of another atom. When electropositive metals and electronegative nonmetals combine, one or more electrons transfer from the metal to the nonmetals by the conversion of cations and anions. The cations and anions produced by such a process must acquire the electronic configuration of the nearest noble gases, and thus they are stable.
The cations and anions thus obtained may attract each other by the electrostatic force of attraction. Therefore, an ionic bond can be defined as the electrostatic force of attraction between a cation and an anion formed by the complete transfer of an electron from an atom of an electropositive element to an electronegative element.
Formation of Ionic Bonds
Electropositive metals, like alkali or alkaline earth metals, have the tendency to lose one or more electrons to form cations.
Na → Na+ + e−
K → K+ + e−
Mg → Mg+2 + 2e−
Ca → Ca+2 + 2e−
Electronegative non-metals, like halogens and chalcogens, have the tendency to gain one or more electrons to form anions.
F + e− → F−
Cl + e− → Cl−
O + 2e− → O−2
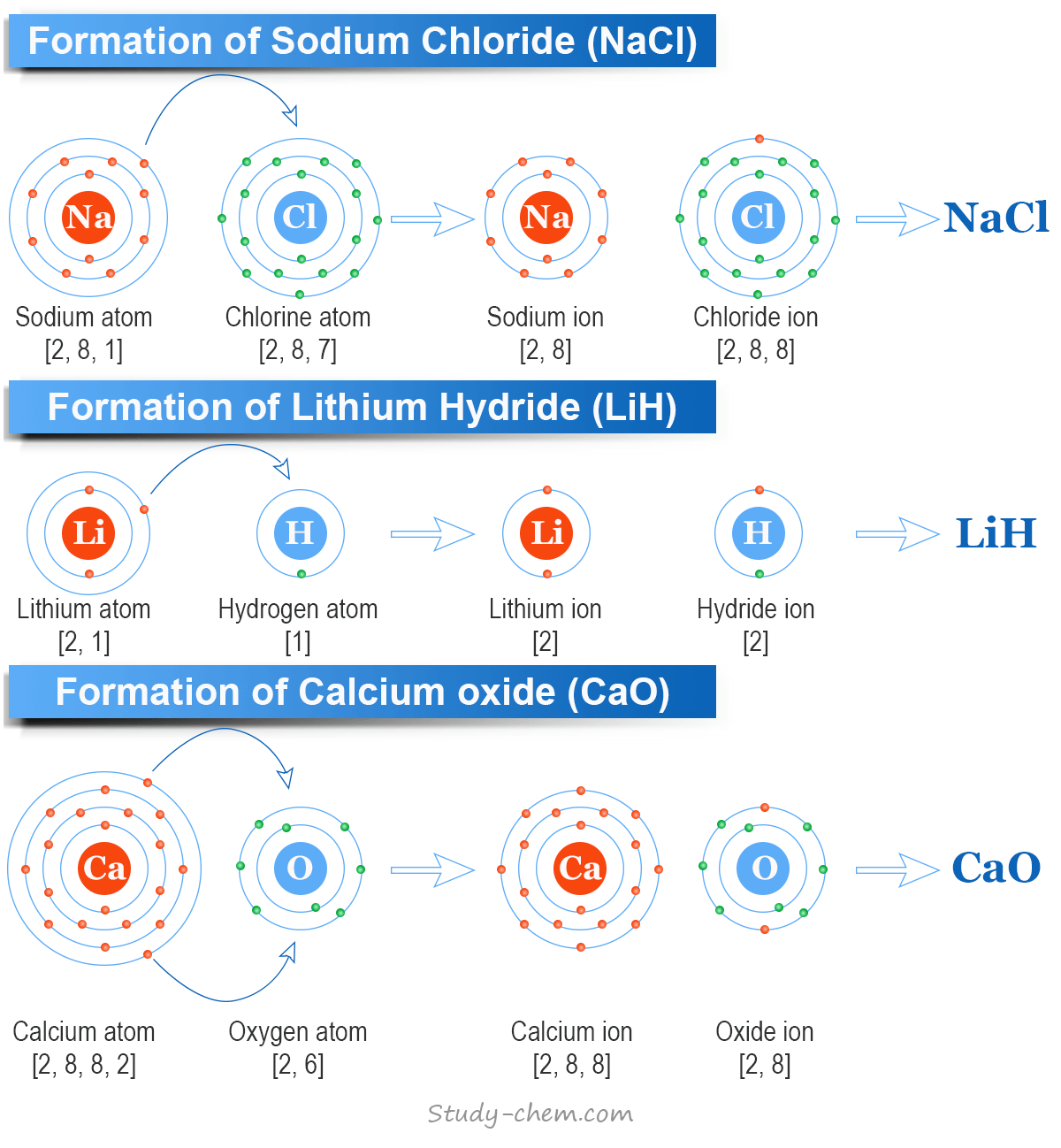
A mutual electrostatic force of attraction is responsible for the formation of ionic bonding in the ionic compounds like NaCl, KCl, LiH, CaCl2, CaO, MgO, etc.
They are readily ionized, conduct electricity, and are formed by the transfer of electrons or electrons from one atom to another.
Formation of Sodium Chloride (NaCl)
The cations and anions always achieve an inert gas electronic configuration when they form ionic bonds or compounds. For example, the formation of a crystalline solid like sodium chloride (NaCl).
- When sodium and chlorine atoms come into contact with each other, the sodium atom loses one electron to achieve the electronic configuration of neon (1s2 2s2 2p6).
- However, the chlorine atom gains this electron to achieve the electronic configuration of argon (1s2 2s2 2p6 3s2 3p6).
- A mutual electrostatic force of attraction between the oppositely charged sodium ion and a chlorine ion is generally responsible for the formation of ionic bonding in sodium chloride (NaCl).
Properties of Ionic Compounds
Ionic compounds formed through ionic bonding exhibit a range of physical and chemical properties. The most common properties of ionic compounds are:
- Non-directional character
- Hardness
- Melting and boiling points
- Conduction of electric current
- Solubility
Non-directional character of Ionic bond
The polar linkages in ionic compounds are non-directional in nature because two oppositely charged ions in ionic compounds just touch each other by a partial neutralization. Therefore, each ion in ionic compounds is surrounded by the greatest possible number of oppositely charged ions.
No separate or discrete molecules are present in the crystal lattice of ionic compounds. Therefore, it exists as a non-directional array of oppositely charged ions. So, formula weight is more appropriate than molecular weight for ionic compounds.
Hardness of Ionic Compounds
The cations and anions in ionic compounds are held together by strong electrostatic forces of attraction. A considerable amount of energy is required to break such strong electrostatic forces of attraction. Therefore, the ionic compounds are hard and stable.
Melting and Boiling Points
A very high melting and boiling point of the ionic crystal lattice occurs due to the formation of an endless array of oppositely charged ions by strong electrostatic forces of attraction. A considerable amount of heat is required to break such strong electrostatic forces of attraction in an ionic crystal lattice.
Conduction of Electric Current
In the solid state, ionic compounds are poor or bad conductors of electricity, while in the fused state or in a solution, these compounds are good conductors of electricity.
- The positive and negative ions present in a solid ionic crystal can fix their position due to strong electrostatic forces of attraction. Therefore, they can not move when an external electric field is applied to them.
- Ionic compounds in their fused state conduct electricity because they are ionizable in the fused state. Therefore, the cations and anions move freely when an external electric field is applied to ionic compounds.
- An ionic solid in water or any other solvent conducts electricity because it ionizes in water or any other solvent to give free ions in solution.
Solubility of Ionic Compounds
Ionic compounds are polar in nature. Therefore, they are generally soluble in polar solvents such as water (H2O), ammonia (NH3), etc. However, they are insoluble in non-polar solvents such as benzene (C6H6), carbon tetrachloride (CCl4), etc.
Formation of Covalent Bond
A covalent bond is generally formed between two atoms by mutual sharing of one, two, or three electron pairs between them. Each of the two combining atoms attains a stable noble gas configuration by such mutual sharing of electron pairs. Each combining atom contributed one electron to form such an electron pair and has an equal claim on the shared electron pair.
A covalent bond may be defined as a force holding atoms together through the sharing of electrons having opposite spins. A pure covalent bond is non-polar and non-ionized because, in its formation, electrons are not transferred from one to the other. Therefore, a molecule having a covalent bond between two similar atoms does not acquire polarity, and no ions are formed. For this reason, a pure covalent bond is also called a nonpolar bond.
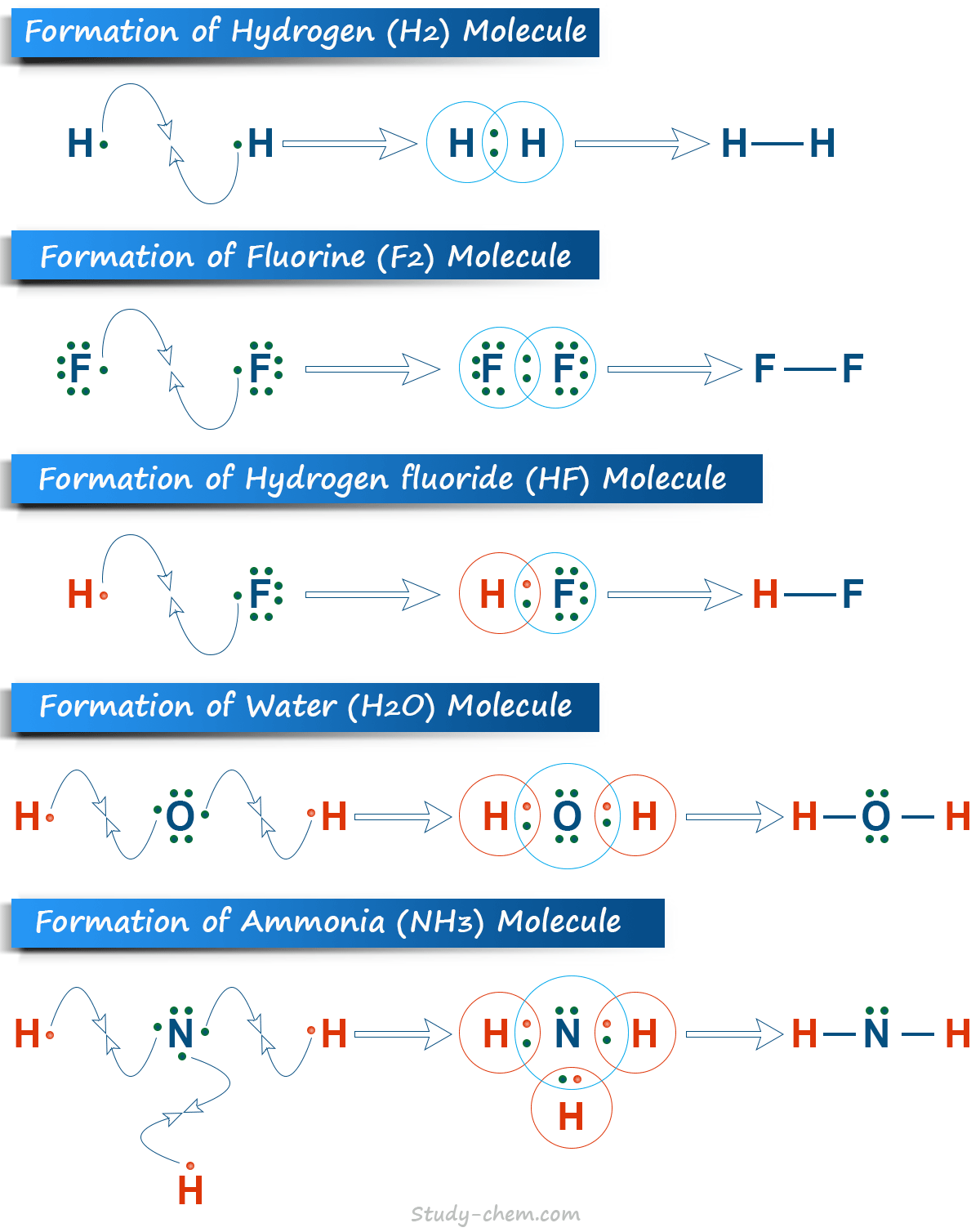
Lewis Electronic Structure of Covalent Compounds
Lewis in 1919 suggested that there are atoms that attain a noble gas configuration by sharing one or more electron pairs with similar or dissimilar atoms. He also provides a method in which Lewis’s electronic structure of covalent compounds can be written.
- For writing Lewis electronic structure, we should choose the central atom that is less electronegative.
- The symbol of all elements is written down in the order in which they are connected.
- A dot or cross symbol can be used for the representation of electrons.
- A small straight line is used to connect the atoms of elements, and a dot or cross line is used to represent unshared electrons.
The Lewis electronic structure for various molecules, such as hydrogen (H2), fluorine (F2), hydrogen fluoride (HF), water (H2O), and ammonia (NH3), is given below in the picture.
Types of Covalent Bond
Covalent bonds are mainly of two types: a single covalent bond and a multiple covalent bond. The multiple covalent bonds are mainly double and triple covalent bonds.
Single Covalent Bond
A single covalent bond may be formed by the sharing of only one electron pair between the bonded atoms. For example, hydrogen, fluorine, chlorine, water, and ammonia are formed by only single covalent bonding.
H· + ·H → H:H → H−H
Double Covalent Bond
A double covalent bond may be formed when the atoms are bonded together by sharing two electron pairs. Among these two bonds, one is a sigma bond, and one is a pi bond. For example, oxygen, ethylene, and carbon dioxide are formed by double covalent bonding.
O: + :O → O::O → O=O
Triple Covalent Bond
A triple covalent bond can be formed when the atoms are bonded together by sharing three electron pairs. Among these three bonds, one is a sigma bond, and two are pi bonds. For example, nitrogen and acetylene are formed by triple covalent bonding.
N⋮ + ⋮N → N⋮⋮N → N≡N
Octet Rule
We see that when two atoms form a covalent bond, they generally attain an inert or noble gas configuration. It is called the octet rule. However, in a hydrogen molecule, each of the two hydrogen atoms contains two electrons with a 1s² configuration.
Incomplete Octet
The central atoms, Be, B, and N in BeCl2, BF3, and NO are surrounded by four, six, and seven electrons in their valence shell, respectively. While other atoms of these molecules obey the octet rule. Therefore, the central atoms of such molecules have an incomplete octet.
Expansion of Octet
Many central atoms of the elements of the third and subsequent periods have more than 8 valence shell electrons. For example, PCl5, ClF3, ICl3, OsF8 have more than 8 valence shell electrons for central atoms P, Cl, I, Os.
- The five chlorine atoms form five covalent bonds with phosphorus in the PCl5 molecule. Therefore, the central atom (Phosphorus) is surrounded by (5+5) = 10 electrons.
- Similarly, in ClF3 and ICl3, there are three covalent bonds and four free electrons in each central chlorine and iodine atom. Therefore, each central atom is surrounded by (6+4) = 10 electrons in these two molecules.
Properties of Covalent Compounds
Covalent compounds formed by the sharing of electrons exhibit a range of physical and chemical properties.
- In a crystal lattice, covalent molecules are held by weak Van der Waals forces.
- Covalent compounds are generally insoluble in water but soluble in organic solvents like benzene, carbon tetrachloride, etc.
- Unlike electrovalent compounds, covalent compounds are solids, liquids, or gases at room temperature. However, they are solids if their molecular weights are high. Therefore, chlorine (mol wt 71) is a gas, bromine (mol wt 160) is a liquid, while iodine (mol wt 254) is a solid.
- They are generally soft, easily fusible, and volatile.
- Due to the absence of free electrons, they are a poor conductor of electricity, but graphite is an exception.
- The covalent bond is directional, and there is a possibility of position and stereoisomerism.
Formation of Metallic Bonding
Metals are generally good electrical and thermal conductors. They also have high refracting, melting, and boiling points. They form crystalline solids with high coordination numbers. Ionic and covalent bonding can not explain such properties of metals.
- All the atoms in a metal crystal are identical. Therefore, they can not bind together by ionic bonding because an ionic bond is formed between atoms that have different electronegativities.
- Very weak Van der Waals forces in covalent bonding can not explain the high melting points of metals. The number of valence electrons may also be insufficient for the formation of covalent bonds in metal crystals.
Electron Sea Model of Metallic Bonding
How the metal atoms in metal bonded together can be explained generally by the electron sea model. Valence bond theory and molecular orbital theory are also used for the explanation of metallic bonding.
The electron sea model was first proposed by Drude in 1900 and modified later by Lorentz in 1916. Therefore, it is also called the Drude-Lorentz theory.
According to the electron sea model, each atom in a metal crystal loses all its valence electrons to form an electron pool. The positively charged metal ions are held together by this electron pool. However, the positively charged metal ions do not float randomly in the sea of electrons, but they have a definite position in the crystal lattice.
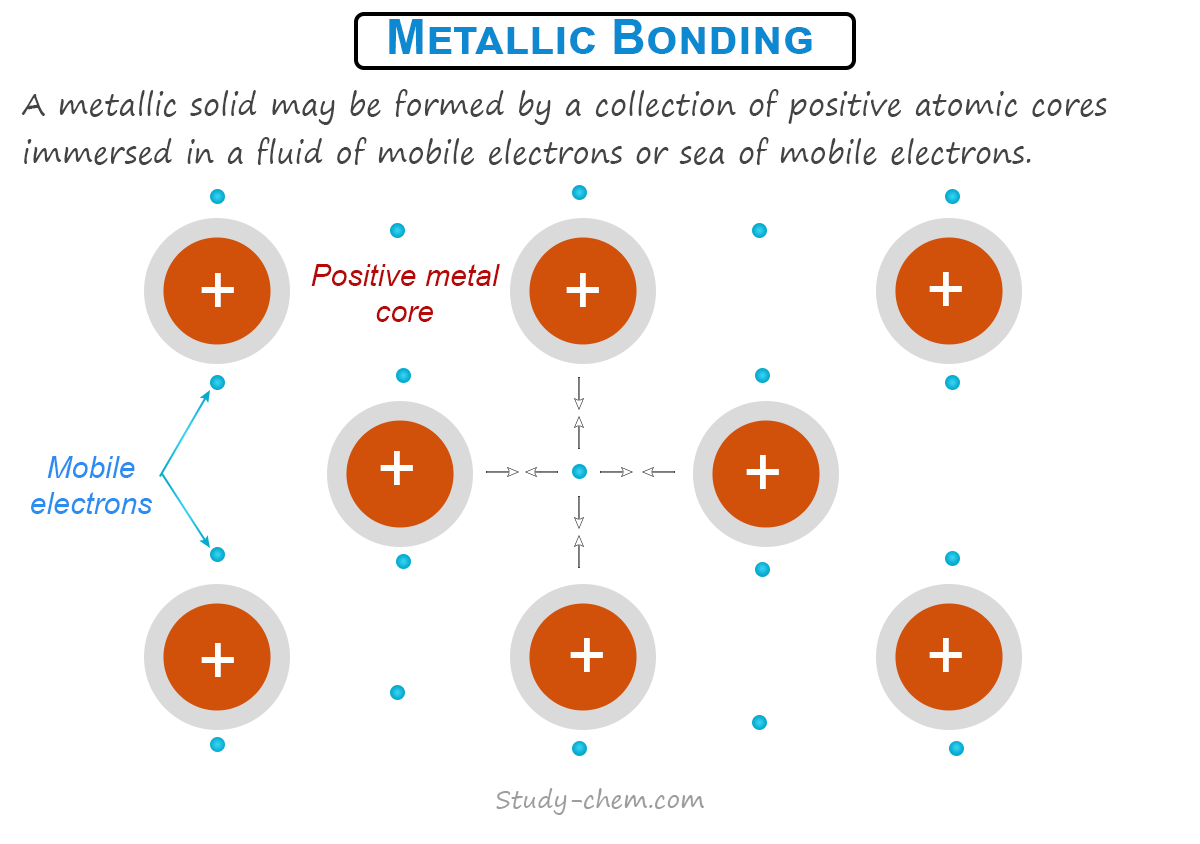
In the electron sea model, the valence electrons in metal crystals are not attached to any individual ions or pair of ions. Therefore, they belong to the crystal as a whole, and they move freely from one part of the lattice to another part. Therefore, according to the electron sea model, metallic solids are a collection of positive atomic cores immersed in a fluid of mobile electrons or a sea of mobile electrons. The metallic bond is a force that binds a metal ion to the mobile electrons within its sphere.
Frequently Asked Questions (FAQs)
What is chemical bonding in chemistry?
A chemical bond in chemistry is an attractive force that holds atoms, ions, or molecules in different species.
What part of the atom is responsible for chemical bonding?
The valence shell electrons, or the electrons in the outermost shell in an atom, are responsible for the formation of chemical bonding. These electrons are generally lost or gained, or shared by an atom to form a chemical bond.
What are the 3 types of bonds?
Chemical bonds are mainly of three types: ionic bond, covalent bond, and metallic bond.
Why do atoms form a chemical bond?
Atoms form a chemical bond to achieve stability or lower energy. Thus, atoms of various elements can be lost or gained, or share electrons to form ionic or covalent bonds.
Define valency of an element.
The number of electrons lost or gained, or shared by an atom of an element to become stable or achieve an octet in the outermost shell, is called the valency of that element.
Why are ionic compounds soluble in water?
Most of the ionic compounds are dissolved in water because they dissociate into their respective ions when they come into contact with water. The process of such dissociation is thermodynamically favorable and kinetically accessible.