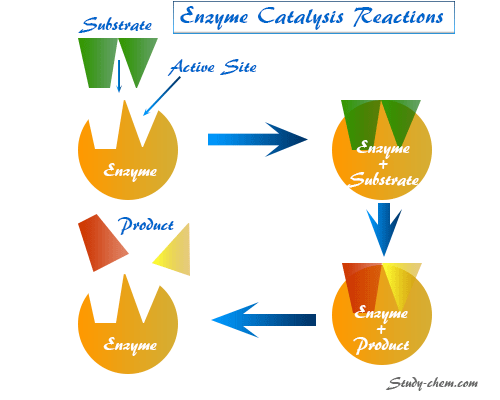
Enzymes Meaning in Science
Enzyme or Enzymes are the colloidal nature of three-dimensional complex protein molecules in science with specific characteristics for the formation of enzyme catalysis reactions. These are produced from the leaving plant and animals from our environment and enzymolysis reactions are examples of kinetics reactions where enzymes act as a catalyst. These are responsible for catalyzing infinite no of chemical changes occurring in the living cells. However, when the enzyme isolated from the cells, they retain their catalytic activity.
Colloidal Nature of Enzyme
In chemistry, enzymes disperse in water forming a colloidal state having dimensions in the colloidal range (10-7 to 10-4 cm). Their kinetic behavior is similar to that of the heterogeneously catalyzed reaction. They provide variously functional groups at the catalytic site that can become equilibrium with the substrate molecule and thereby catalyze a chemical reaction. Therefore the enzyme catalysis reactions have often been referred to as micro heterogeneous zero-order kinetic reactions.
Examples of Enzyme Catalysis Reactions
Enzyme catalysis reactions of enzymes are highly specific in nature. For example, the urase can catalyze and hydrolysis of urea but it does no effect on the hydrolysis of methyl urea. Diastase converts starch to maltose and maltase converts it’s to glucose. This is on further action with enzyme zymase to form alcohol.
Specific Characteristics of Enzymes
The enzyme is the copolymers of amino acids with specific amino acid sequences. The enzyme is poisoned and activated like other heterogeneous catalysts. The activity of enzymes increases experimentally with the rise in temperature but when the temperature is significantly raised, they lose their activity as they coagulate. The activity formed maximum with temperature rise 35 to 45 centigrade.
Kinetics of Enzymolysis Reaction
The enzymes are very effective catalysts also in the different laboratory processes. The concentration of the enzyme required is very much lower than that of the substrate. The rate of enzymolysis is found to be directly proportional to the concentration of enzymes. When the concentration of substrate varied, enzymolysis reactions usually formed the initial first-order chemical kinetics at low substrate concentration and approaches zero-order kinetics when substrate concentration increases.