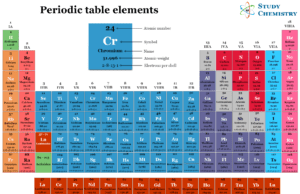
Chemical Element
chemical element is a substance that cannot be decomposed into simpler substances by the common chemical processes in chemistry or chemical science. All the periodic table elements are distributed throughout the entire universe and their isotopic compositions have been estimated by a wide range of chemical and spectroscopic studies on the sun and solar systems like stars, galaxies, nebulae, and interstellar space.
Element is the fundamental chemical material of matter or chemical compound now extends up to 112, with the scopes for further addition.
In this article, we study the origins, abundance, and chemical behavior or facts of the periodic table element. Only 75 elements occur in the earth’s crust in a concentration greater than 10-4 ppm. The average relative content of an element in any natural system is called its abundance.
Spectroscopic analysis of the celestial bodies and analysis of materials provided knowledge about the abundance of elements over the whole universe or cosmos (cosmic abundance). The abundance of the elements on the earth is somewhat different from cosmic abundance.
Each abundance data (earth, atmosphere, and surface water) is helpful in providing systematic scientific information about a chemical element with respect to its formation, properties, stability, and occurrence.
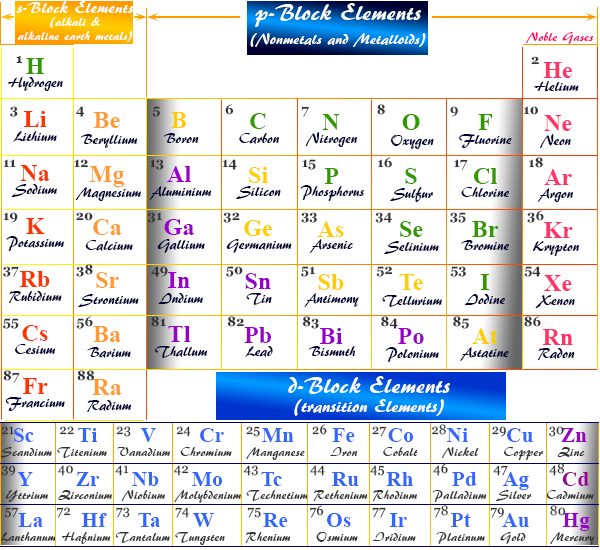
Origins of Chemical Element
A very small fraction of the mass of the earth’s crust (hydrosphere and atmosphere) is available for direct analysis and study of the composition, facts, and chemical properties of an element.
Systematic data and other studies primarily describe that the earth consists of several zones based on the density of naturally occurring chemical elements. The several types of earth zones consist, of the crust (∼36 kilometers in the depth of earth), the mantle (36 to 2900 kilometers), and the core (2900 kilometers to the center of the earth).
Primarily the crust describes a heterogeneous layer of silicates and oxides of different chemical elements, the mantle has a fairly homogeneous combination of different types of silicates, mainly of magnesium and iron.
Geophysical evidence indicates that the core should contain elements with the properties of molten iron and some nickel (mean atomic number = 22). It also composes some lighter chemical elements like sulfur, carbon, silicon, and oxygen.
Occurrence of Elements
The abundance of different types of chemical elements depends on the mode of occurrence or basic units of substances.
Some elements present in the crust in considerable amounts never occur in any concentrated form, pure form of an element dispersed from common minerals. These elements are defined as dispersed elements of the environment. For example, copper is much less abundant than rubidium. Therefore, rubidium is dispersed in potassium minerals, similarly, gallium is dispersed in aluminum minerals but the chemical element chromium and lead are comparatively less abundant periodic table metals.
The availability of chemical elements depends largely on their chemical compounds or minerals composition. Chemically extraction of the element from such dispersed states is impractical for technical and economic reasons.
The main forms of existence of common chemical elements on earth’s crust form the compounds like silicates, carbonates, sulfides, and sulfates.
According to Goldschmidt, chemical elements are classified into four groups siderophile or iron-loving, chalcophile or copper-loving, lithophilic or stone-loving, and atmophilic or vapor-loving periodic table elements.