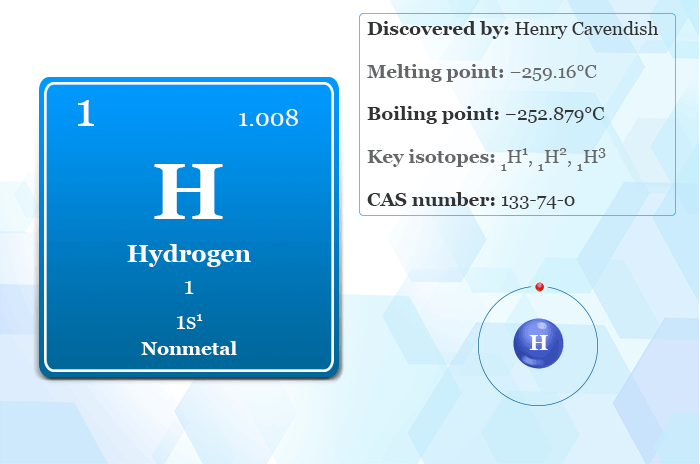
What is Hydrogen?
Hydrogen, symbol H, molecular formula H2 is a colorless, odorless, tasteless, flammable gaseous chemical substance in the periodic table. In chemistry or chemical science, the hydrogen atom is the only member of the chemical element in which the valence electron is under the direct influence of the nucleus. It bearing one unit of positive and negative electrical charge.
Under ordinary conditions, hydrogen gas consists of a pair of atoms or a diatomic molecule with a wide range of bonding. The most important chemical compound water (H2O) is obtained by burning it with oxygen molecules.
Properties of Hydrogen
Due to its unique characteristics and properties, hydrogen (H) forms chemical compounds or molecules with nearly every periodic table element.
| Hydrogen Facts |
|||
| Symbol | H | ||
| Discovery | Henry Cavendish (1766) | ||
| Origin of the name | The name is derived from the Greek word ‘hydro’ and ‘genes’ meaning water forming | ||
| State at 20°C | Gas | ||
| Relative atomic mass | 1.008 | ||
| CAS number | 133-74-0 | ||
| Periodic Properties |
|||
| Atomic number | 1 | ||
| Electron per shell | 1 | ||
| Electron configuration | 1s1 | ||
| Group | 1 | ||
| Period | 1 | ||
| Block | s-Block | ||
| Physical Properties |
|||
| Melting point | −259.16°C | ||
| Boiling point | −252.879°C | ||
| Density (g cm−3) | 0.000082 | ||
| Atomic properties |
|||
| Oxidation states | −1, +1 | ||
| Atomic radius (Å) | 1.10 | ||
| Covalent radius | 0.32 | ||
| Electron affinity (kJ mol−1) | 72.769 | ||
| Electronegativity (Pauling scale) |
2.20 | ||
| Ionisation energies (kJ mol−1) | 1st | 2nd | 3rd |
| 1312.05 | – | – | |
| Main isotopes | 1H, 2H, 3H | ||
The normal oxidation number or state of hydrogen in chemical compounds is +1 but highly electropositive metals (alkaline and alkaline earth), show a −1 oxidation state.
Hydrogen in Periodic Table
In the modern periodic table, hydrogen is placed in group-1 with alkali metals.
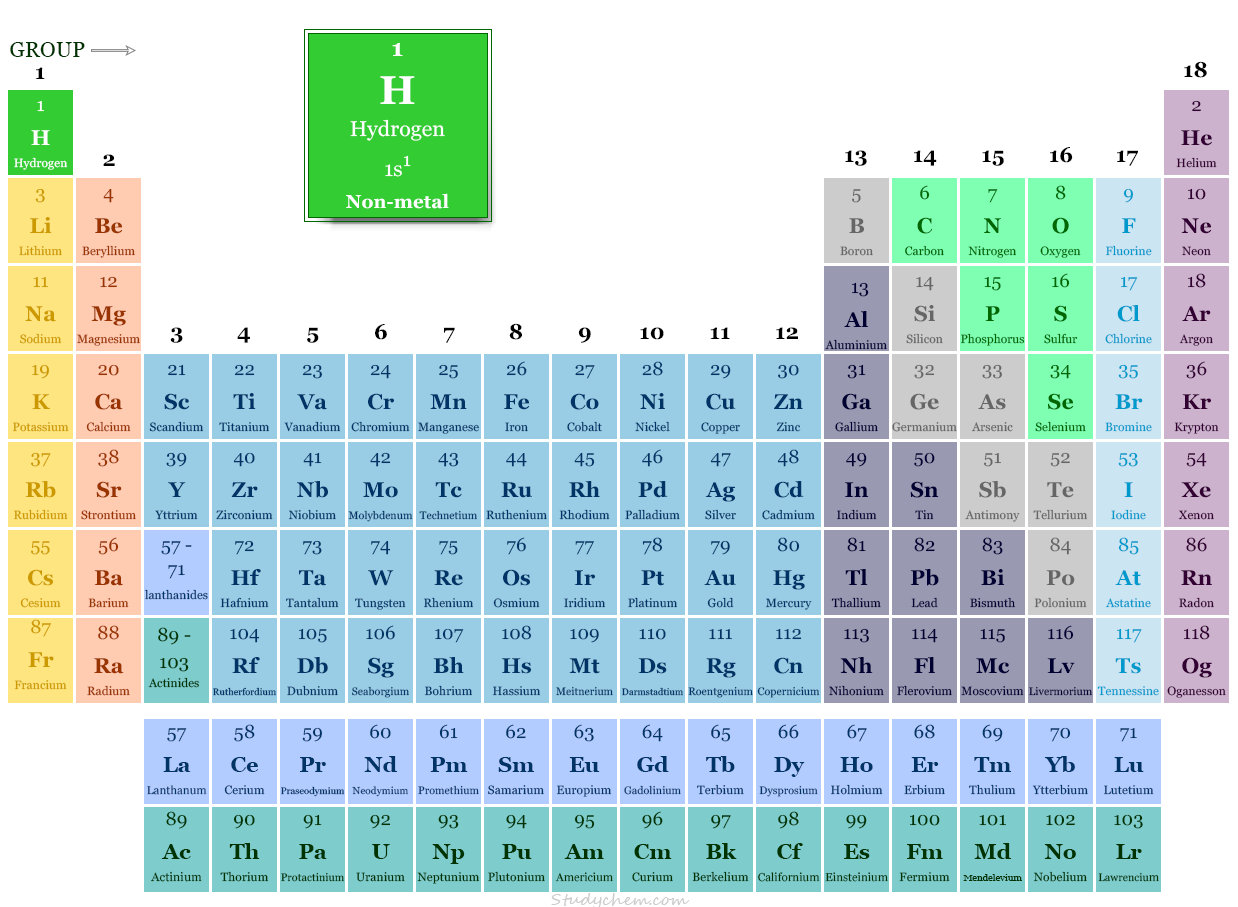
The unique periodic table position can be described by its characteristics properties and electron configuration.
- The ns1 electron configuration justifies its position in group-1 with the alkali metals.
- Considering other facts, the electronic configuration of hydrogen is one electron short of the next noble gas helium (He). Therefore, it is placed in group-17 in the periodic table with the halogen.
- Due to the presence of a half-filled valence shell, it is also placed in group-16 with the carbon family. It forms covalent bonds with a wide range of polarities in chemistry.
Properties of Hydrogen Gas
The chemistry of hydrogen has late gained. It uses as an alternative source of energy in the near future (fuel cells) due to the huge stock of H2 in the earth’s surface water molecules.
The molecule is a very stable species, at 2000°K and 1-atmosphere pressure, only one percent of atomic hydrogen is present in the gas molecules. Therefore, these facts suggested the lack of reactivity at ordinary temperature and pressure.
Among atomic forms, it forms various unstable ionized species like a proton (H+), a hydride ion (H−), and a molecular ion (H2+).
Hydrogen Isotopes
The three isotopes of hydrogen gas molecules are represented as H2 (dihydrogen), D2 (di-deuterium), and T2 (di-tritium). The chemical properties of all these gas molecules are basically the same but hydrogen isotopes differ in their physical properties. At standard temperature and pressure, H2 gas exists as a colorless, orderless, tasteless, highly combustible, non-toxic, nonmetallic chemical compound.
Preparation Formula
Hydrogen is prepared
- by the action of metals on water and acids
- by the electrolysis of water
- the reforming hydrocarbon by the stream.
It is also obtained as a byproduct in the electrolytic manufacture of caustic potash from brine.
Small Scale Preparations
The alkali and alkaline earth metals like sodium (Na), calcium (Ca), magnesium (Mg), and aluminum (Al) react with water in cold conditions evolving hydrogen gas. In hot conditions, metals like Zn, Fe, Co, Ni, and Mn decompose water.
Large Scale Production
Very pure H2 and O2 can be obtained from the electrolysis of 20 percent caustic soda or potash solution in steel cells using iron electrodes. The anode is nickel plated to prevent oxidation. The anode and cathode are separated by a porous diaphragm to prevent diffusion and mixing of the gases.
Large amounts of H2 molecules are now obtained from the reforming of natural gas (hydrocarbon) by steam in the presence of a nickel catalyst at 900°C.
CnH2n+2 + nH2O → nCO + (2n+1)H2
Some carbon dioxide and methane are also formed by this process. The gas mixture cooled and removed carbon dioxide (CO2) and methane(CH4) for the fresh preparation of H2 gas molecules.
Uses of Hydrogen Gas
- H2 is used for the manufacture of chemicals like ammonia, hydrochloric acid, alcohols (methanol), aldehyde (formaldehyde), etc.
- The atomic, molecular, and liquid form is also used in fuel cells (hydrogen-oxygen fuel), hydrogenation of oils, welding atomic torches, reduction of metal ores, and propulsion of rocket (in Saturn-V).
- In bubble chambers, it is used for the study of high-energy particles.
Uses of Liquid Hydrogen
In the near future, liquid hydrogen is used as an alternative source of energy when our reserves of fossil fuel (coal, oils, and natural gas) will get exhausted.
The great advantage of liquid hydrogen is used as fuel. It is free from the pollution hazard of conventional fuels.
The only problems lie with the storage and transmission of large quantities of gases. Therefore, vacuum-isolated cryogenic tanks, it used to store huge quantities of liquid hydrogen in the United States, Canada, Germany, and the United Kingdom space programs.