Periodic Table Elements in Chemistry
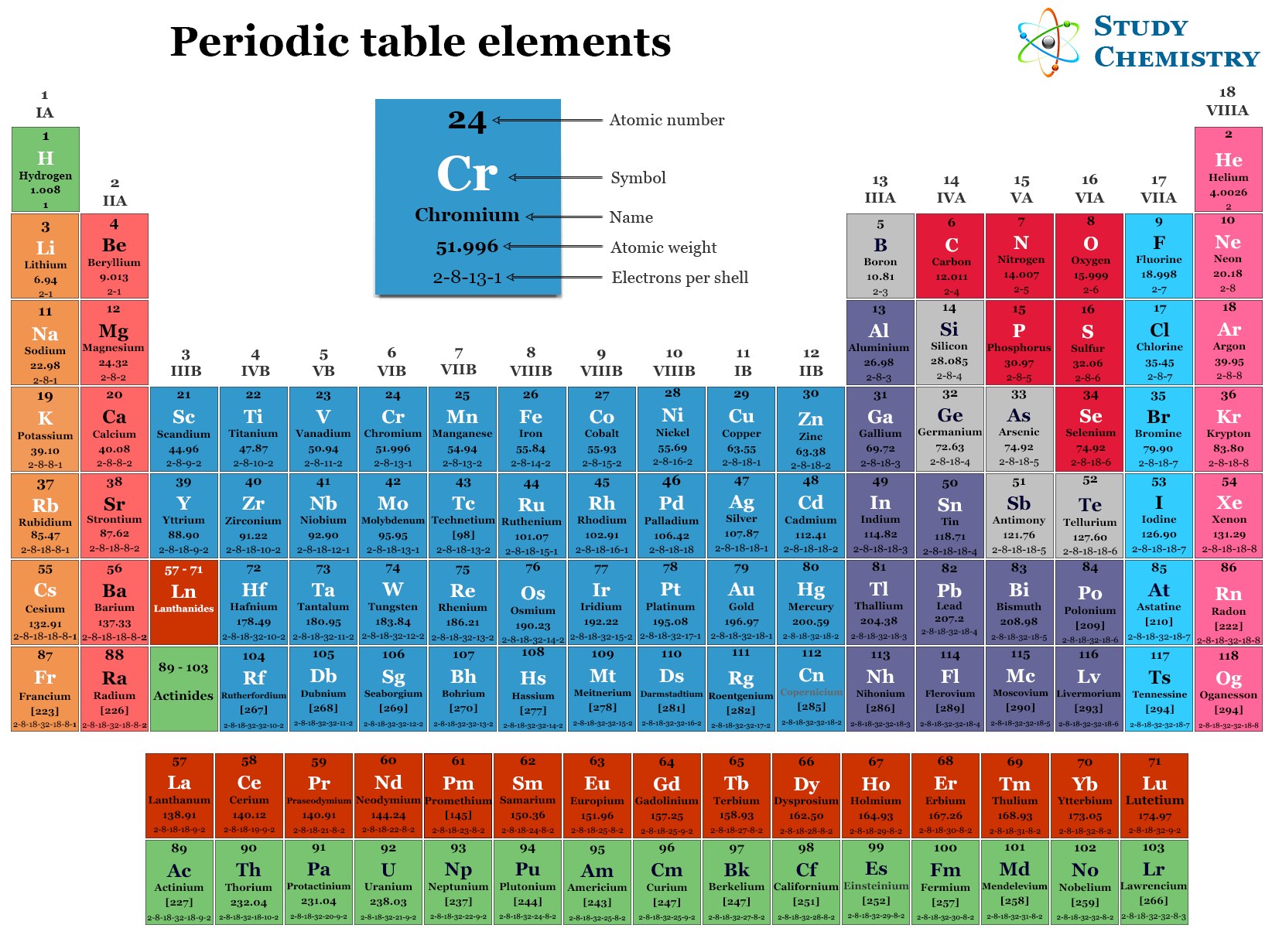
The periodic table (modern and long form) provides a tabular display of the chemical elements arranged according to their properties, such as the atomic number and electronic configuration. It is called periodic because the elements having similar properties are repeated after certain intervals or periods. Modern periodic law generally classifies chemical elements based on their atomic number or electronic configuration. The name, symbol, atomic number, atomic weight, and electronic configuration of 118 periodic table elements are given below the table.
Mendeleev’s periodic law
A German chemist, Lother Meyer, states that the physical properties of the elements are periodic functions of their atomic masses. However, in 1861, Mendeleev first attempted to classify the chemical elements according to their chemical properties.
He derived the law known as Mendeleev’s periodic law, which states that the physical and chemical properties of the elements are a periodic function of atomic weight. Mendeleev’s periodic table divides the chemical elements according to groups or vertical columns and periods or horizontal rows.
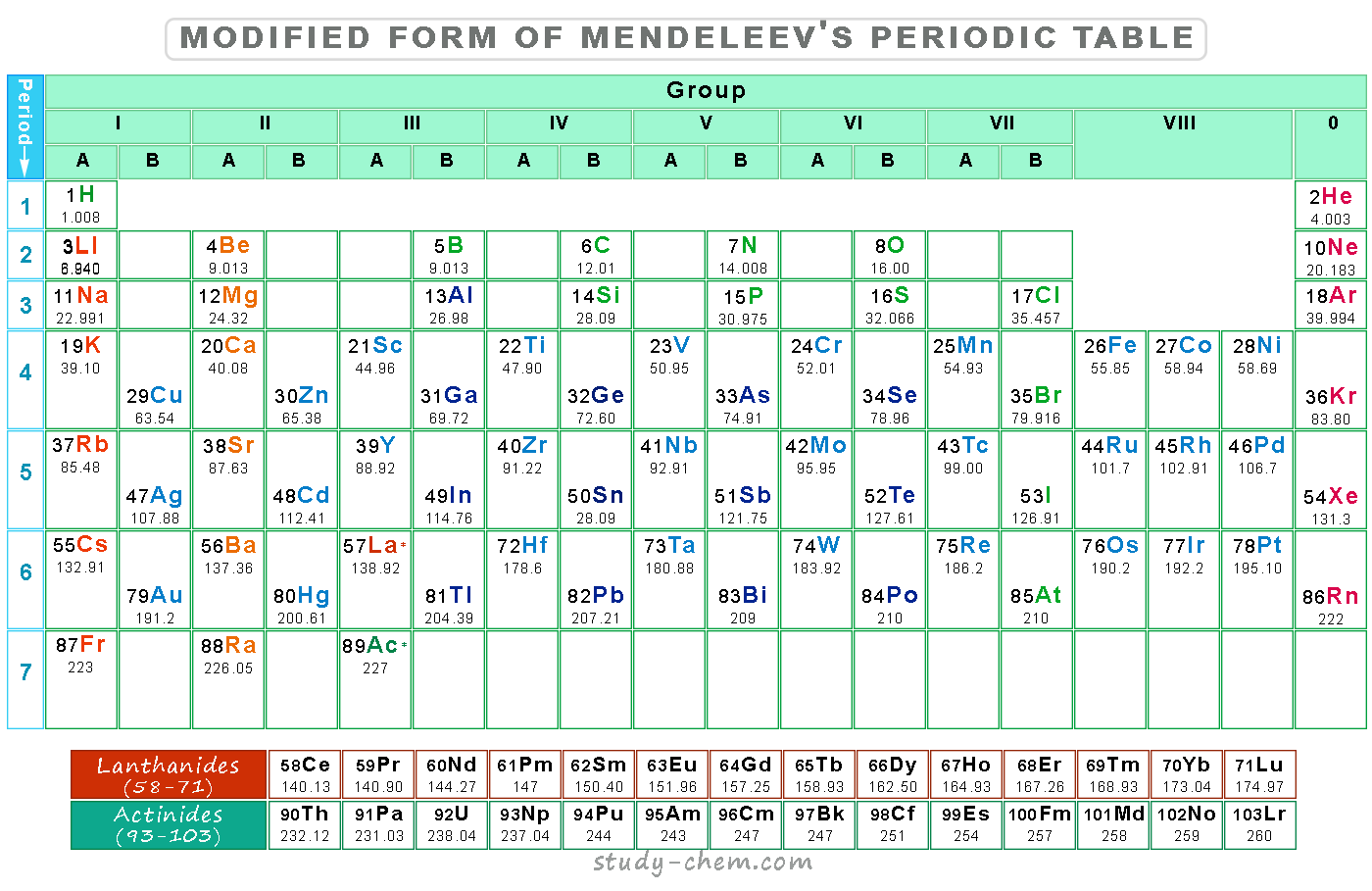
Mendeleev’s statement about the periodic table needed to be changed in view of Moseley’s research. The position of three pairs of periodic table elements, like tellurium and iodine, argon and potassium, nickel and cobalt, was not arranged according to their chemical behavior.
For example, cobalt has an atomic weight of 58.9 amu, and nickel has 58.7 amu. Hence, according to Mendeleev’s law, cobalt should be placed after nickel, but the atomic number of cobalt is equal to 27, and nickel is equal to 28. The accommodation of rare earths and isotopes on the basis of atomic weight also causes problems.
| Element | Atomic Weight (amu) |
| Nickel (Ni) | 58.69 |
| Cobalt (Co) | 58.94 |
| Argon (Ar) | 39.94 |
| Potassium (K) | 39.10 |
| Tellurium (Te) | 127.61 |
| Iodine (I) | 126.91 |
Modern Periodic Law
The modern law of the periodic table is based on Moseley’s work. It explains most of the general properties of elements on the basis of their atomic number.
The modern periodic law states that the physical and chemical properties of chemical elements are a periodic function of their atomic number.
Long Form of Periodic Table
In order to remove the defects of the Mendeleev tabular display, a number of tables have been suggested for the classification of chemical elements. All the tabular displays are based on the modern periodic law. Therefore, the elements are arranged in order of increasing atomic number.
Out of such tables, one of the most widely used forms is the long form of the periodic table or Bohr’s table. It is a periodic chart based on the electronic configuration of elements.
In the long form of the periodic table, the elements are classified into four types, such as s-block, p-block, d-block, and f-block, according to their valence shell electronic configuration.
Periods in the Modern Periodic Table
Periods are the horizontal rows present on the periodic table. The long form of the modern periodic table contains seven periods.
| Period 1 | 1 H |
2 He |
||||||||||||||||
| Period 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||
| Period 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||
| Period 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ga |
33 As |
34 Se |
35 Br |
36 Kr |
| Period 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
| Period 6 | 55 Cs |
56 Ba |
57 La |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
| Period 7 | 87 Fr |
88 Ra |
89 Ac |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Cn |
113 Nh |
114 Fl |
115 Mc |
116 Lv |
117 Ts |
118 Og |
| Lanthanides | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Ed |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
|||
| Actinides | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
|||
- The first period contains two periodic table elements with only one main energy level. It is called a very short period.
- The second period contains eight chemical elements from lithium (Li) to neon (Ne). It is called a short period.
- The third period also contains eight periodic table elements from sodium (Na) to argon (Ar). It is also called a short period.
- The fourth period has 18 elements from potassium to krypton. It is called a long period.
- The fifth period also has 18 elements from rubidium to xenon. It is also called a long period.
- The sixth period has 32 elements, and it forms a very long period.
- The seven-period also has 32 elements. Such a very long period generally contains radioactive elements.
Groups in the Modern Periodic Table
Groups in the periodic table are the vertical columns in which chemical elements are arranged according to their properties. There are 18 groups or vertical columns in the modern or long form of the periodic table.
- Group 1 and group 2 contain alkali and alkaline earth elements. Generally, they are highly reactive metals in the periodic table.
- The group 3 to group 12 elements are called heavy metals or transition metals.
- Most of the elements present in group 13 to group 17 are generally non-metals. They can react easily with metals present in the table.
- The inert gases or noble gases are placed in group 18 of the periodic table. They are generally inert to any chemical reactions.
Defects of the Long Form of the Periodic Table
The position of hydrogen: The position of hydrogen in the long form of the periodic table can not be solved completely. However, the valence shell electronic configuration of hydrogen is similar to that of alkali metals, but their properties are not similar.
Position of Helium: The chemically inert helium possesses 1s2 configuration, but other inert gases possess ns2np6 configuration. Therefore, a different place should be given to helium.
Helium and alkaline earth metals also have similar valence shell electronic configurations. Therefore, helium can be placed in the group of alkaline earth metals of the periodic table. But this can not be done because the properties of helium and alkaline earth metals are completely different.
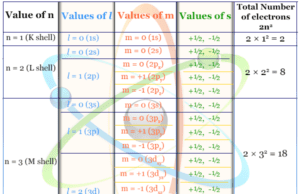
Classification of Elements on the Basis of Electronic Configuration
On the basis of electronic configuration, periodic table elements are classified into four types: s, p, d, and f-block elements. It depends on the nature of the atomic orbitals into which the last electron enters.
s-Block Elements
The extreme left portion of the long form of the periodic table generally contains highly electropositive elements like alkali metals and alkaline earth metals. Such group 1 (alkali) and group 2 (alkaline earth metals) can constitute the s-block of the periodic table.
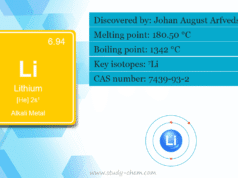
The valence shell electronic configuration of group 1 metals (H, Li, Na, K, Rb, Cs, and Fr) is ns1. However, the valence shell electronic configuration of group 2 metals (Be, Mg, Ca, Sr, Ba, and Ra) is ns2.
p-Block Elements
The right potion consists of the elements of group 13 to group 18 (noble gases). Generally, all nonmetals, some metals, and all the metalloids are present in this portion of the long form of the periodic table.
- Group 13 (Boron Family): The elements in the boron family contain B, Al, Ga, In, and Tl.
- Group 14 (Carbon Family): The elements in the carbon family contain C, Si, Ge, Sn, and Pb.
- Group 15 (Pnictogens): The elements in pnictogens contain N, P, As, Sb, and Bi.
- Group 16 (Chalcogen): The elements in chalcogens contain O, S, Se, Te, and Po.
- Group 17 (Halogens): The elements in halogens contain F, Cl, Br, I, and At.
- Group 18 (Inert or Noble Gases): The elements in inert or noble gases contain B, Al, Ga, In, and Tl.
The noble gases are present in the extreme right; however, extensively electronegative elements or halogens are situated on the left of the noble gases. Only the exception of He, whose electronic configuration (1s2) is a member of this block.
The electrons in atoms of these periodic table elements enter 2p, 3p, 4p, 5p, and 6p orbitals, respectively. Since s-orbitals in these atoms are already filled. Therefore, the valence shell electronic configuration of the atoms of these elements varies from ns2np1 to ns2np6.
d-Block Elements
In d-block elements, the electron enters the (n−1)d orbital of the (n−1)th main energy level. These chemical elements are placed in the middle (group 3, 4, 5, 6, 7, 8, 9, 10, 11, 12) in the periodic table. Therefore, they lie between s and p-block elements.
The valence shell electronic configuration of these elements is (n−1)d1→10ns0, 1, or 2. These elements are also called transition elements. They are classified into four series, such as 3d, 4d, 5d, and 6d.
- 3d series: Such a series has ten elements, such as Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, and Zn.
- 4d series: This series also contains ten elements, such as Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd.
- 5d series: These series also contain ten elements from La to Hf. Here, valence electrons are entered in 5d orbitals.
- 6d series: Such a series also has ten elements from Ac to Cn. While most of these elements in this series were discovered recently.
f-Block Elements
In the f block of the periodic table, the extra electrons enter the (n−2) f orbits of the (n−2) th main shell. Therefore, the valence shell electronic configuration of these elements is (n−2)fo→14(n−1)d0, 1, or 2ns2.
The atoms of these elements have three incomplete outer shells. The f-block elements generally constitute two series, the 4f series or lanthanides and the 5f series or actinides. These three incomplete shells are the outermost ns orbital, (n−1)d orbital, and (n−2)f orbital.
- The 4f series or lanthanide series has 14 elements from Ce (58) to Lu (71).
- Similarly, the 5f series or actinide series also has 14 elements from Pa (91) to Lr (103).
Periodic Trends of Chemical Elements
Periodic trends are a specific pattern of the properties of chemical elements when we proceed from left to right across a period and from top to bottom in a group of the periodic table. The most common periodic trends include electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character.
Atomic Radius
The term atomic radius generally used to derive the distance between the nucleus and the outermost shell of electrons of the atoms of the periodic table elements. It is a periodic function of the atomic number. Therefore, atomic number changes along a group and a period of the periodic table.
- In a Period: On moving from left to right in a period, the electrons are added to the orbitals of the same main energy level. It means the number of electrons and protons in the atoms of elements increases, but the outermost shell remains the same. Therefore, outer electrons are attached more strongly to the nucleus, resulting in a decrease in atomic radius.
- In a Group: When we move down in a group of the periodic table, the atomic radius of elements increases due to the addition of a new electronic shell. However, the addition of a new electronic shell may increase the distance between the nucleus and the outermost electron. Therefore, the electrostatic attraction between the nucleus and valence shell electrons may decrease. Such decreased electrostatic attraction increases the atomic radius of the elements.
Inosation Energy
Ionization energy is the amount of energy required to remove the most loosely bound electron or outermost electron of an isolated gaseous atom of an element to produce a cation.
The ionization energy trends of periodic table elements generally vary along a group and along a period. Various factors such as nuclear charge, completely filled and half-filled orbitals, shielding or screening effect, and atomic radius generally influence the magnitude of ionization energy and its periodic trends.
Generally, when we move from left to right in a period ionization energy of elements increases. Increase in ionization energy across a period generally explained by a decrease in atomic radius and an increase in effective nuclear charge.
Therefore, the ionization energy becomes maximum for noble gases due to their stable filled electronic configuration. However, it is very low for alkali metals due to the presence of the ns1 configuration.
When we move down in a group, the ionization energy generally decreases due to an increase in atomic radius and a decrease in effective nuclear charge.
Electronegativity
The electronegativity of a bonded atom simply defined as a tendency or ability to attract the shared electron pair towards itself. Electronegativity is a periodic property, and it varies along a group and a period.
- Electronegativity increses when we move in a period of the periodic table from left to right. It is due to the increase in nuclear charge from left to right in the periodic table.
- However, when we move down in a group of the periodic table, the nuclear charge increses but electronegativity generally decreases. It generally happened due to an increase in atomic radii. A small atoms attract electrons more strongly than a larger one, therefore they are more electronegative.
Electron Affinity
The electron affinity is the amount of energy released when an electron is added to an isolated gaseous atom of the periodic table elements in its lowest energy state to form an anion. The magnitude of electron affinity also influenced by a number of factors such as atomic size, effective nuclear charge, and screening effect.
- When we move down in a group, electron affinity generally decreases due to a steady increase in the atomic radius of elements.
- However, electron affinity values generally increase on moving from left to right in a period of the periodic table.
Metallic character
The element having a higher value of electronegativity will be essentially a non-metal, while one with a lower value of electronegativity will be a metal. However, a higher value of ionization energy and a lower value of electron affinity will be essentially for a non-metal.
- Therefore, when we move from left to right in a period, the metallic character of the periodic table elements decreases.
- However, when we move from top to bottom in a period, the metallic character of periodic table elements increses.
Therefore, we understand why francium (Fr), which is situated at the bottom left-hand corner of the periodic table, is the most radioactive metal. Similarly, fluorine (F) is the most reactive non-metal present at the top right-hand corner.
Oxidizing and Reducing Properties
When we move from left to right across a period of the periodic table, the atomic radius decreases and electronegativity increses. Therefore, the oxidizing power of the element increses and the reducing power decreases.
The alkali metals have very low ionization energies, and they are strong reductants. Whereas halogens have very high ionization energy but are highly electronegative, and they are strong oxidizing agents.
When we move down in a group, the size of the atom increses and the ionization energy decreases. Therefore, the reducing power of the element increses while the oxidizing power decreases.
Hence, the reducing power of group 1 elements,
Cesium (Cs) > Rubdium (Rb) > Pottasium (K) > Sodium (Na) > Lithum (Li)
On the other hand, the oxidizing power of group 17 or halogens,
Florine (F) > Chlorine (Cl) > Bromine (Br) > Iodine (I)
Frequently Asked Questions (FAQs)
How many chemical elements are present in the 5th period of the periodic table?
The 5th period of the periodic table contains 18 elements. These are two 5s-block, six 5p-block, and ten 4d-block elements.
How many elements are present in the long form of the periodic table?
The periodic table contains 118 chemical elements distributed in 7 periods and 18 groups.
Why do elements in a group of similar properties?
The elements in a similar group have similar properties because they have similar valence shell electronic configuration. Therefore, they have a similar number of electrons in their valence shell, which participate in chemical bonding.
What are the first 20 periodic table elements?
The first 20 periodic table elements are:
- Hydrogen (H)
- Helium (He)
- Lithium (Li)
- Berylium (Be)
- Boron (B)
- Carbon (C)
- Nitrogen (N)
- Oxygen (O)
- Flurine (F)
- Neon (Ne)
- Sodium (Na)
- Magnesium (Mg)
- Aluminum (Al)
- Silicon (Si)
- Phosphorus (P)
- Sulfur (S)
- Chlorine (Cl)
- Argon (Ar)
- Potassium (K)
- Calcium (Ca)
What is the modern law of periodic table elements?
The modern law of the periodic table is based on Moseley’s work. He explains most of the properties of elements on the basis of atomic number, not atomic weight. Therefore, the modern periodic law, based on Moseley’s work, states that the physical and chemical properties of elements are a periodic function of their atomic number.
How many blocks are in the periodic table?
According to the electronic arrangement of the atoms, there are four blocks in the modern or long periodic table, namely s-block, p-block, d-block, and f-block.
How many metals, non-metals, and metalloids are present in the modern or long form of the periodic table?
Among the 118 periodic table elements, most of the elements contain metallic properties. Therefore, the modern periodic table contains 93 different types of metal, 18 non-metals, and 7 metalloids.
Who made the modern periodic table?
The modern law of the periodic table is based on Moseley’s work. The work of Mosley can well explain most of the anomalies and defects of Mendeleev’s periodic table.
How are the chemical elements ordered in the modern periodic table?
The anomalies and defects of Mendeleev’s periodic table disappear when the basis of classification of periodic table elements changed from atomic weights to atomic numbers. Therefore, elements ordered on the basis of atomic number in the modern periodic table.
How many groups are in the modern periodic table?
There are 18 groups and 7 periods in the modern and long form of the periodic table, in which all 118 chemical elements are arranged.